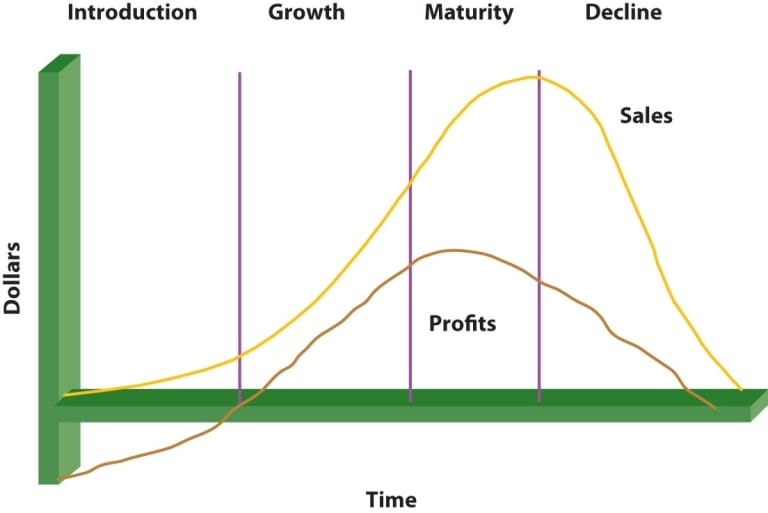
Becoming an outstanding product manager in the pharmaceutical industry and creating a top-tier brand demands a blend of strategic insight, industry expertise, and flawless execution. Here’s a comprehensive guide to help you excel and establish a number-one product in the market. 1. Deeply Understand Your Market and Audience To create a leading product, you need to understand the therapeutic area thoroughly. Stay updated on the latest research, clinical trials, and market trends that affect your product’s development. Equally important is understanding your target audience—patients, healthcare professionals, regulators, and payers. Recognizing their needs and behaviours is critical for crafting a tailored product and marketing strategy. 2. Conduct Robust Market Research Market research is your foundation. Start with competitor analysis to understand the gaps and areas for differentiation. Investigate unmet patient needs by gathering insights directly from patients and healthcare providers through surveys and focus groups. Additionally, staying informed about the regulatory landscape in your target markets will help ensure compliance and smooth market entry. 3. Develop a Compelling Value Proposition Your product’s unique selling proposition (USP) should be clear and compelling. It could be superior efficacy, a better safety profile, or more affordable pricing. Define what makes your product stand out in the market and create a brand promise that aligns with both the scientific benefits and emotional value your product provides to patients and healthcare providers. 4. Cross-Functional Collaboration Effective collaboration across departments is essential. Work closely with R&D to ensure that your product addresses unmet needs and is scientifically sound. Marketing, sales, and clinical teams should be aligned to maintain consistent messaging and create a strong, unified brand presence in the field. 5. Create an Effective Launch Strategy A successful launch is a phased process. First, ensure your product’s market entry strategy is carefully planned, taking into account the competitive landscape, pricing, distribution, and regulatory approvals. Building strong clinical evidence and cultivating relationships with key opinion leaders (KOLs) will help position your product as an industry leader from the start. 6. Focus on Innovation and Continuous Improvement Innovation doesn’t end with the product launch. Focus on patient-centric innovations such as support programs, adherence tools, and digital solutions that enhance the overall patient experience. Always plan for product lifecycle management—explore line extensions, new indications, and post-launch strategies to maintain the product’s relevance. 7. Embrace Digital and Data-Driven Marketing In today’s digital age, leveraging digital marketing is key. Invest in strategies that allow you to engage healthcare professionals and patients directly. Use data analytics to optimize your marketing spend and track performance. Digital tools like apps and patient support programs can also help improve adherence, creating stronger brand loyalty. 8. Build and Protect Your Brand Consistency is crucial in building a trustworthy brand. Ensure that all your communications, from marketing materials to customer interactions, reflect a unified brand identity. Post-market surveillance allows you to monitor product feedback, address issues, and refine your approach as needed. 9. Stay Agile and Resilient The pharmaceutical industry is dynamic, with frequent changes in regulations, market trends, and new breakthroughs. Stay adaptable, ready to pivot when necessary. Have a crisis management plan in place for unforeseen events such as negative clinical results or product recalls. 10. Leadership and Influence Strong leadership is crucial in steering cross-functional teams toward common goals. Develop your ability to inspire and align teams with a shared vision. Networking with industry influencers and thought leaders can open doors for new collaborations, enhancing the credibility and reach of your product. By focusing on these principles, you can build a standout brand that resonates with both healthcare professionals and patients, and ultimately achieve your goal of creating a number-one brand in the pharmaceutical space. If you’re looking for ways to enhance your product’s impact and drive stronger results, exploring effective communication strategies can make all the difference.